Turkey’s Ministry of Environment, Urbanization and Climate Change has issued draft implementation rules under KKDIK, its national chemicals regulation modeled on the EU REACH framework. The proposal introduces concrete deadlines for pre-registration, Lead Registrant (LR) nomination, transitional registrations, and Safety Data Sheet (SDS) updates—marking a significant step forward in regulatory clarity for the Turkish chemicals market.
1. Pre-Registration Deadlines Announced
Companies manufacturing or importing chemicals ≥1 tonne/year must pre-register via Turkey’s KKS system and join the MBDF (Substance Information Exchange Forum) by 30 June 2025.
For substances introduced after this date, pre-registration deadlines will be staggered by tonnage band:
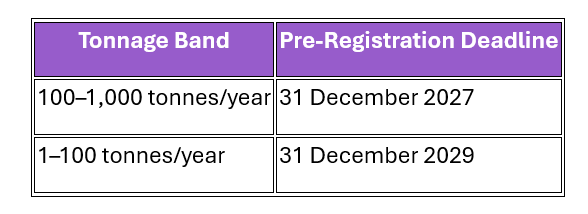
|
2. Lead Registrant Nomination Deadline
Each substance registered under KKDIK must have a Lead Registrant (LR) appointed by 30 September 2025.
🗳️ Nomination threshold: Requires approval from 70% of participating companies
🔁 Re-validation: Previously nominated LRs must undergo re-approval under the new process
📡 Role of LR: Coordinates dossier submissions and facilitates intra-consortium communication
3. Transitional Registration Requirements
The draft rule outlines a detailed registration timeline:
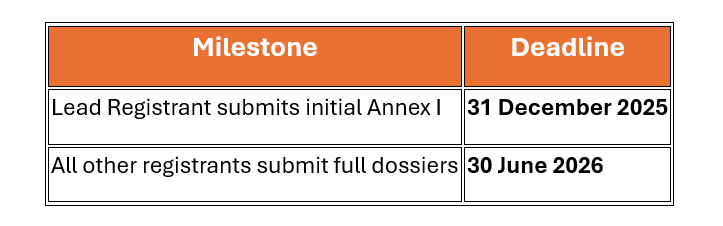
|
✅ Optional Fast-Track: Companies submitting complete registrations by year-end 2025 may bypass the transitional pathway.
💸 Fees Apply: Transitional submissions will incur applicable regulatory fees under KKDIK.
4. Safety Data Sheet (SDS) Compliance
📑 SDS Updates Required: Suppliers must upload KKDIK-compliant SDSs to Turkey’s centralized SDS platform.
• Must align with Turkish formatting and GHS-based hazard classification
• SDSs must be prepared in Turkish and updated according to KKDIK Annex II guidelines
Strategic Advantages of KKDIK Implementation
The draft rules mark a major advancement in Turkey’s chemical regulatory landscape:
✅ Regulatory Certainty: Industry now has clear, actionable deadlines for compliance
🔄 Operational Preparedness: Enables resource planning and consortium coordination
🌍 Global Alignment: Supports Turkey’s broader harmonization with EU REACH standards
Implications for Industry Stakeholders
Companies manufacturing, importing, or distributing chemicals in Turkey should:
📋 Catalogue substances by tonnage to determine registration requirements
🤝 Engage early in MBDF forums ahead of the 30 June 2025 pre-registration deadline
🧩 Consider becoming a Lead Registrant, particularly larger or data-owning firms
📆 Plan for transitional submissions (late 2025–mid-2026), including budgeting for dossier preparation and fees
🗂️ Update SDS systems and ensure documentation complies with Turkish regulatory language and format standards
Reach out to our regulation experts on chemical and product regulatory compliances

