Committee: Biocidal Products Committee (BPC)
Date of Decision: June 2025
Next Step: Final adoption by the European Commission and Member States
The European Chemicals Agency’s (ECHA) BPC has recommended the approval of several anticoagulant rodenticides for continued use under the EU Biocidal Products Regulation (BPR). These substances are critical to rodent control but raise concerns due to persistence, bioaccumulation, and non-target species exposure.
Approved Active Substances
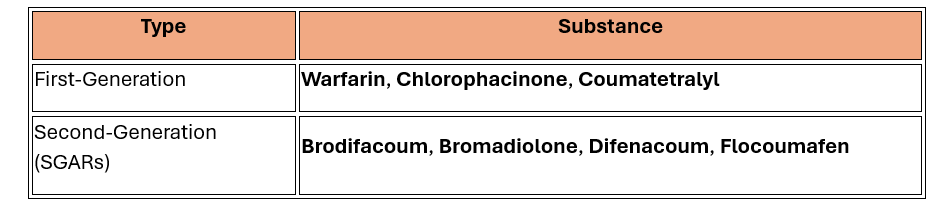
|
These approvals cover both professional and non-professional use, subject to strict conditions.
Risk Management Measures (RMMs)
To address environmental and human health risks, the following RMMs are expected:
❗ Restrictions on outdoor use to limit exposure to non-target wildlife
🧠 Mandatory training for professional users
🛡️ Specialized packaging and labeling requirements
🔄 Promotion of Integrated Pest Management (IPM) practices:
o Rodenticides to be used only after non-chemical options prove insufficient
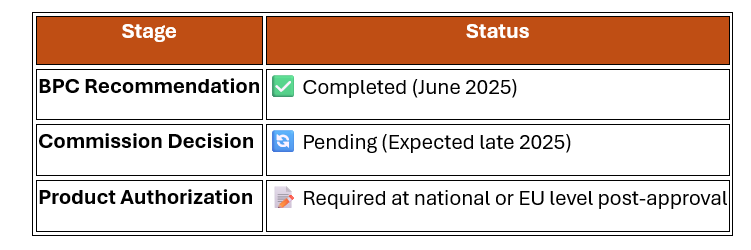
|
Manufacturers and distributors must apply for product-level authorization once final decisions are made.
Industry Implications
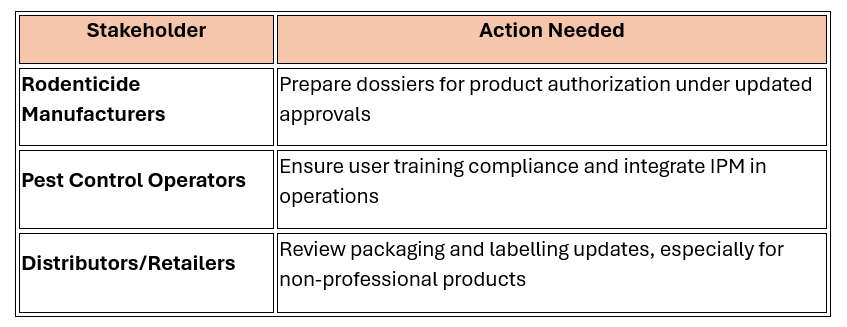
|
Strategic Importance
This marks a significant regulatory milestone for the pest control industry, balancing public health needs with the EU’s sustainability and biodiversity protection goals. The BPC’s decision reinforces the principle of "last resort" use for chemical rodenticides under the broader push for sustainable biocidal practices.
Reference: ECHA: BPC Backs Approval of Anticoagulant Rodenticides
Reach out to our regulation experts on chemical and product regulatory compliances

