Mandatory IUCLID Use for Biocide Renewal Applications
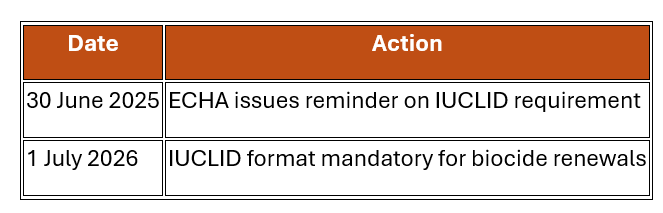
On 30 June 2025, the European Chemicals Agency (ECHA) issued a notice that starting 1 July 2026, all renewal applications for biocidal active substances under the Biocidal Products Regulation (BPR) must be submitted using the IUCLID format. This step enhances data consistency and regulatory efficiency across the EU.
Why IUCLID Matters?
IUCLID (International Uniform Chemical Information Database) is a standardized digital format widely used for chemical data under EU regulations like REACH and BPR. Its adoption for biocide renewals offers:
• Harmonized, structured data submissions
• Faster, clearer regulatory evaluations
• Better integration with global chemical databases
Who Must Comply?
Companies holding active substance approvals for biocides that need renewal by mid-2026 must convert their dossiers into IUCLID format to meet the new submission rules.
Industry Guidance: Preparing for Compliance
ECHA advises companies to start preparations immediately by:
• Reviewing existing renewal dossiers
• Updating data within the IUCLID platform
• Using the IUCLID Validation Assistant to check completeness and format accuracy
Taking early action helps avoid submission delays or rejection due to format issues.
Key Dates to Remember
|
Tips for a Smooth Transition
• Begin IUCLID dossier conversion well before deadlines
• Utilize available guidance and tools from ECHA
• Seek expert assistance if unfamiliar with IUCLID preparation
Reference: ECHA: Biocide renewal applications in IUCLID
Reach out to our regulation experts on chemical and product regulatory compliances

