EU Seeks Feedback on Hazard Classification of Chemicals under CLP Regulation
As of June 2025, the European Chemicals Agency (ECHA) has launched public consultations on several proposals for Harmonised Classification and Labelling (CLH) of chemical substances under the EU CLP Regulation (EC No 1272/2008). These consultations invite input from industry, NGOs, academics, and the general public on proposed hazard classifications for various substances.
🔍 Purpose of the CLH Consultation Process
• Ensure harmonised hazard communication across EU Member States
• Support scientific risk assessment of substances
• Promote consistency in classifying hazards such as:
o Carcinogenicity
o Reproductive toxicity
o Skin sensitisation
o Environmental and acute toxicity
Even if a dossier submitter suggests no classification, the Risk Assessment Committee (RAC) may recommend one based on available data.
💬 How Stakeholders Can Contribute
• General Comments: Substance identity, properties, or clarifications
• Hazard Class Comments: Only accepted for classes open to public input
o Can address classification rationale, omitted data, or misinterpretations
• RAC may:
o Agree or disagree with proposals
o Suggest stricter or more lenient classifications
o Propose substance-specific concentration limits or subgroup classifications
📋 Active CLH Consultations – June 2025
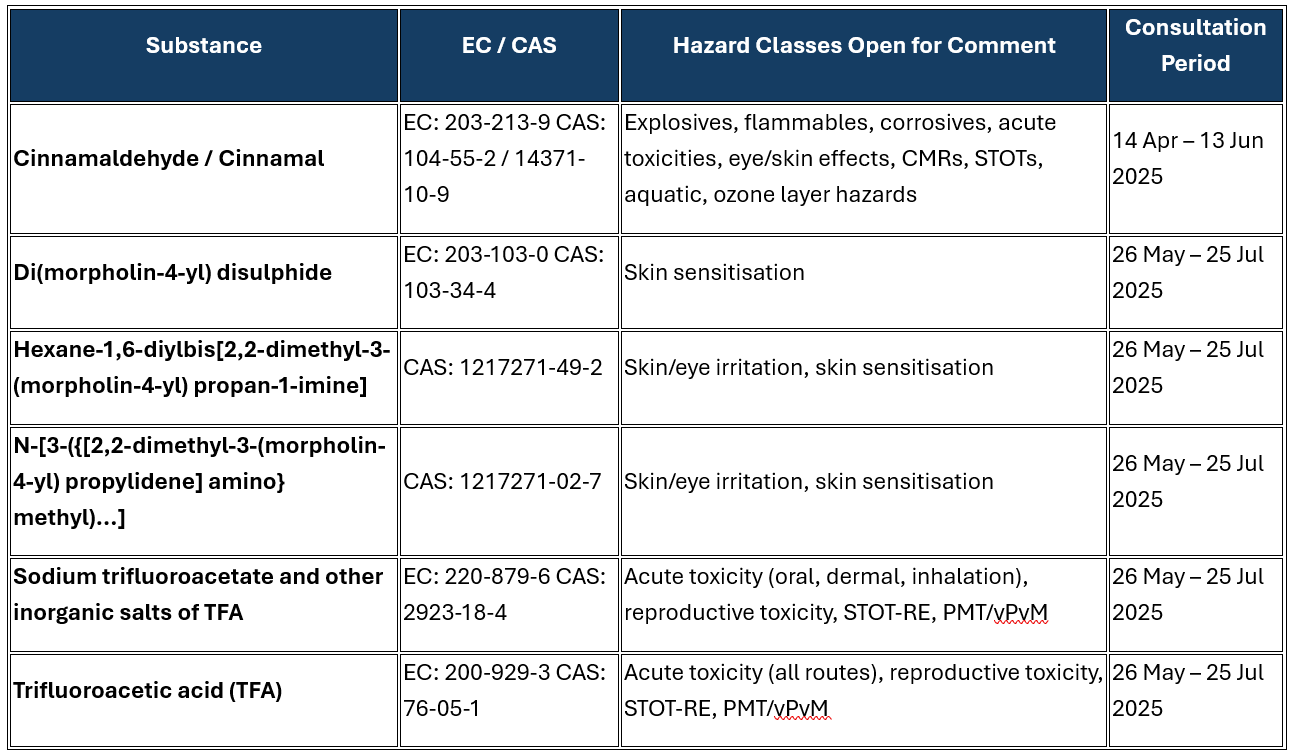
|
Next Steps:
Following the consultation, the RAC will review submitted data and comments, potentially adjusting classifications before formal adoption into CLP Annex VI.
For details or to submit comments, visit ECHA’s CLH consultation portal.
Reference: ECHA – CLH Consultations, June 2025
Reach out to our regulation experts on chemical and product regulatory compliances

