The Medical Device Coordination Group (MDCG) released updated guidance—MDCG 2024-14 Rev. 1—clarifying how contact lenses must comply with the Master UDI-DI requirements under the EU Medical Device Regulation (MDR).
The revision introduces a new legal reference, an extended compliance deadline, and links to supplementary resources to ease industry transition.
Key Updates in the 2025 Revision
📜 New Legal Reference
• Adds Commission Delegated Regulation (EU) 2025/788, which officially delays the Master UDI-DI requirement for contact lenses.
• Updates the reference link to the consolidated MDR regulation.
⏳ Extended Transition Period
• New compliance deadline: 9 November 2026
• Previous deadline was in 2025
• Manufacturers now have an extra year to fully align their UDI systems and technical documentation
🔗 Additional References
• Refers to MDCG 2025-7, explaining timelines for highly individualized devices (HIDs)
• Includes EU resource page for UDI requirements for HIDs
Summary of Original Guidance (Still Valid)
The guidance continues to clarify how contact lenses should be grouped and tracked using UDI elements:
🔍 Definitions
• Differentiates between standard and made-to-order (MtO) contact lenses
• Explains UDI elements:
o Basic UDI-DI
o Master UDI-DI
o UDI-PI (Production Identifier)
📦 Assignment & Labelling Rules
• Master UDI-DIs must be assigned based on design features like power, diameter, and base curve
• Guidance on labelling formats (AIDC & HRI) and packaging levels
📝 Device Registration
• Clarifies how to register contact lenses in EUDAMED, including rules for legacy devices
⚠️ Vigilance Reporting
• In case of incidents, manufacturers must use complete UDI information to ensure accurate device traceability
Who Is Affected and How?
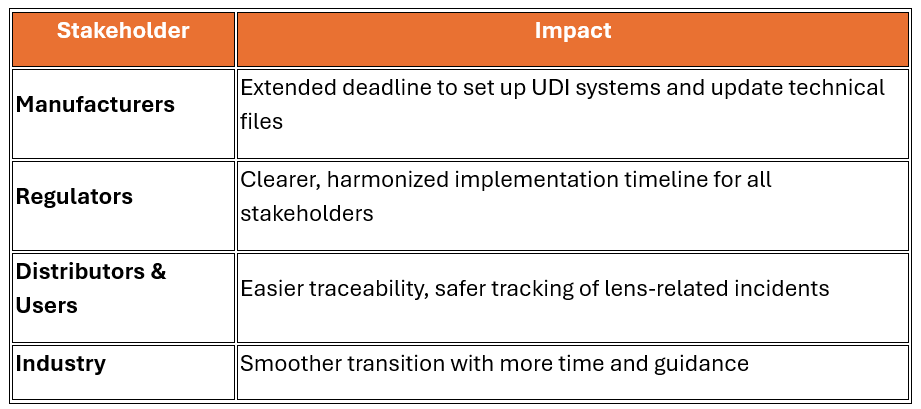
|
Next Steps for Companies
✅ Review the Updated Guidance: Carefully read MDCG 2024-14 Rev. 1 to understand UDI grouping, labeling, and reporting expectations
✅ Revise Compliance Timelines: Adjust internal planning to meet the new 9 November 2026 compliance date
✅ Update Technical Documentation: Ensure UDI grouping, labelling, and packaging match the updated rules
✅ Register in EUDAMED: Follow required steps for entering contact lenses into the EU database
✅ Prepare for Vigilance Requirements: Ensure incident reporting tools and SOPs include full UDI details
✅Use Additional Resources: Refer to MDCG 2025-7 and the HID UDI portal for broader regulatory alignment
This revision brings much-needed clarity and flexibility to contact lens manufacturers. The one-year extension to November 2026 provides an opportunity to finalize systems and documentation. However, early action is still essential to ensure smooth compliance under the EU MDR.
Reference: MDCG 2024-14 Rev.1 – Master UDI-DI for Contact Lenses (PDF)
Reach out to our regulation experts on chemical and product regulatory compliances

