Entry into Force: Regulation (EU) 2025/1234 enters into force on 16 July 2025
The European Commission has adopted Implementing Regulation (EU) 2025/1234, significantly amending the existing eIFU Regulation (EU) 2021/2226. The revised regulation broadens the eligibility for electronic instructions for use (eIFU) to include nearly all medical devices intended for professional use, including non-medical purpose products listed in Annex XVI of the MDR and legacy devices.
What’s New – Expanded Scope and Simplification ?
 |
Practical Implications for Manufacturers
1. Devices Now Eligible for eIFU:
i. All classes of professional-use medical devices ii. Annex XVI devices (e.g., aesthetic products with no direct medical purpose) iii. Legacy devices under MDR transitional provisions
2. Use of eIFU Remains Optional, But...
i. Manufacturers may implement eIFU but must offer a free paper version within 7 days upon request. ii. If the product is likely to be used by non-professionals (e.g., in home care), a paper IFU must be provided by default.
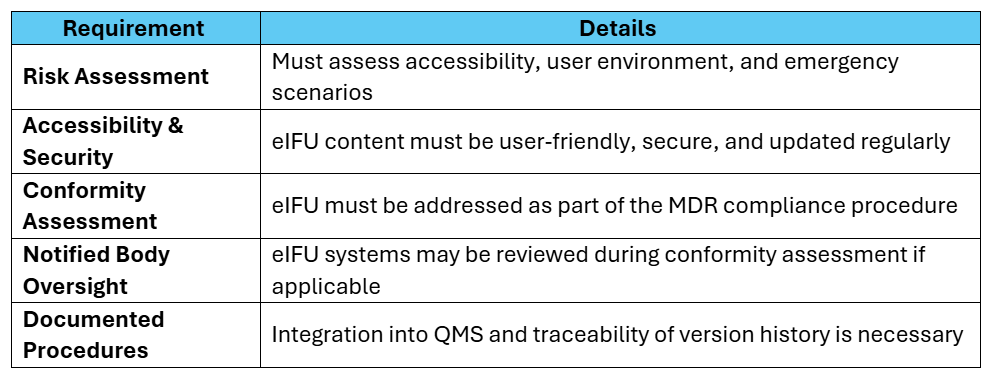
Compliance Requirements Still Apply
Though several administrative requirements are eased, manufacturers must still:
 |
Strategic Recommendations
For Manufacturers Already Using eIFU:
a. Extend coverage to new eligible devices (legacy, Annex XVI, Class I–III) b. Update internal SOPs to reflect reduced documentation and notification obligations
For Manufacturers Not Using eIFU:
a. Evaluate cost-benefit of digital IFU solutions b. Plan for:
Digital infrastructure development Cybersecurity and access control Risk assessment and QMS updates
Consider pilot rollout for low-risk Class I or II devices
Timeline & Enforcement
 |
References:
European Commission Press Release Regulation (EU) 2025/1234 amending (EU) 2021/2226
Reach out to our regulation experts on chemical and product regulatory compliances