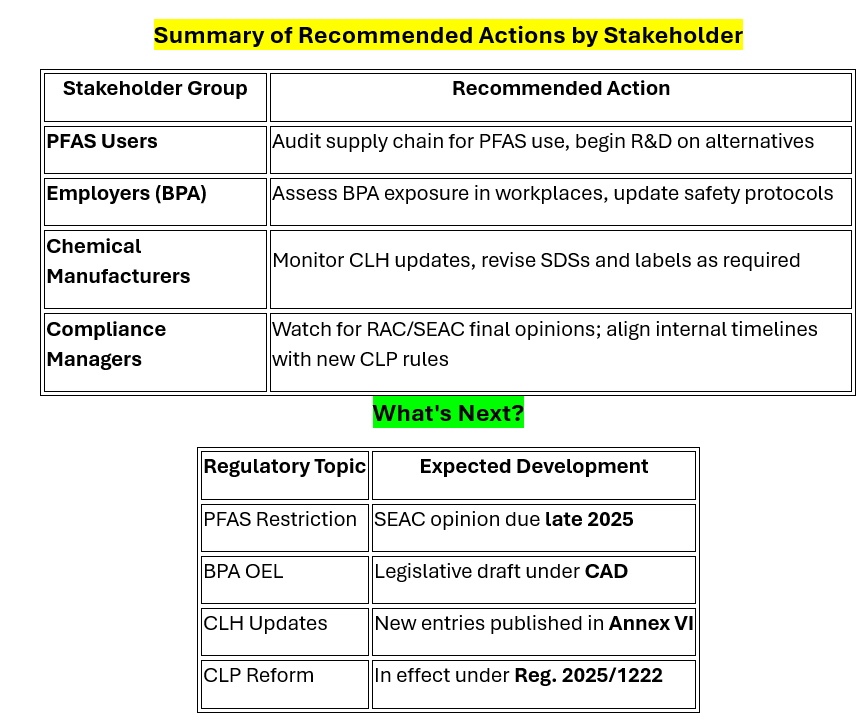
1. PFAS Restriction Proposal: Major Step Forward
• RAC Position: the EU-wide restriction of thousands of PFAS based on their persistent, bioaccumulative, and toxic (PBT) properties.
• SEAC Status: Socio-economic analysis is ongoing. Final opinion expected by end of 2025.
🔍 Implications:
• Broad restriction likely by 2026.
• Affects textiles, food packaging, automotive, medical, electronics, and more.
✅ Action for Industry:
• Begin PFAS substitution planning and supply chain impact assessments.
• Engage with suppliers about PFAS-free alternatives.
2. Bisphenol A (BPA) – Binding OEL Recommended
• RAC Recommendation: Introduced a binding occupational exposure limit (OEL) for BPA, based on its endocrine-disrupting and reproductive toxicity.
• Next Step: Feed into updates under the Chemical Agents Directive (CAD).
🔍 Implications:
• Industries using BPA (plastics, coatings, electronics) must prepare for airborne exposure limits in workplaces.
✅ Action for Employers:
• Evaluate workplace BPA exposure levels.
• Plan updates to ventilation, PPE, and training protocols ahead of CAD adoption.
3. CLP Regulation – Harmonised Classification & Labelling (CLH) Updates
• Changes Approved:
o New or revised classifications for substances with CMR (carcinogenic, mutagenic, reprotoxic) or environmental toxicity.
o Addition of group classifications for structurally similar substances.
• New entries added to Annex VI of CLP Regulation.
🔍 Implications:
• Affects Safety Data Sheets (SDS), product labels, and chemical registration dossiers across the EU.
✅ Action for Chemical Manufacturers:
• Review all products against updated Annex VI classifications.
• Update labels, SDSs, and customer communications accordingly.
4. Regulatory Reform: CLP Classification Procedures (Reg. 2025/1222)
• Key Changes:
o Group submissions now encouraged for related substances.
o Greater transparency: All proposals must be pre-notified and published.
o Defined timelines: RAC opinions and Commission decisions now subject to clear deadlines.
🔍 Implications:
• More predictable classification process.
• Increased visibility of substances under regulatory review.
✅ Action for Stakeholders:
• Track classification proposals early.
• Engage during public comment periods.
• Prepare compliance updates ahead of legal changes.
|
References:
ECHA – June 2025 RAC & SEAC Meeting HighlightsEUR-Lex – CLP Procedure Reform Regulation (EU) 2025/1222
Reach out to our regulation experts on chemical and product regulatory compliances

