The European Union has introduced Regulation (EU) 2025/877, amending Regulation (EC) No 1223/2009 on cosmetic products to further strengthen consumer safety. Effective 1 September 2025, this regulation prohibits the use of newly classified CMR substances (Carcinogenic, Mutagenic, or Toxic for Reproduction) in cosmetic formulations across the EU internal market.
This update aligns the Cosmetics Regulation with CLP Regulation (EC) No 1272/2008 and Delegated Regulation (EU) 2024/197, ensuring uniform protection from harmful substances.
Key Updates Under EU 2025/877
🆕 New Substances Added to Annex II (Prohibited List)
The following substances, recently classified as CMRs under EU 2024/197, are now banned in cosmetic products:
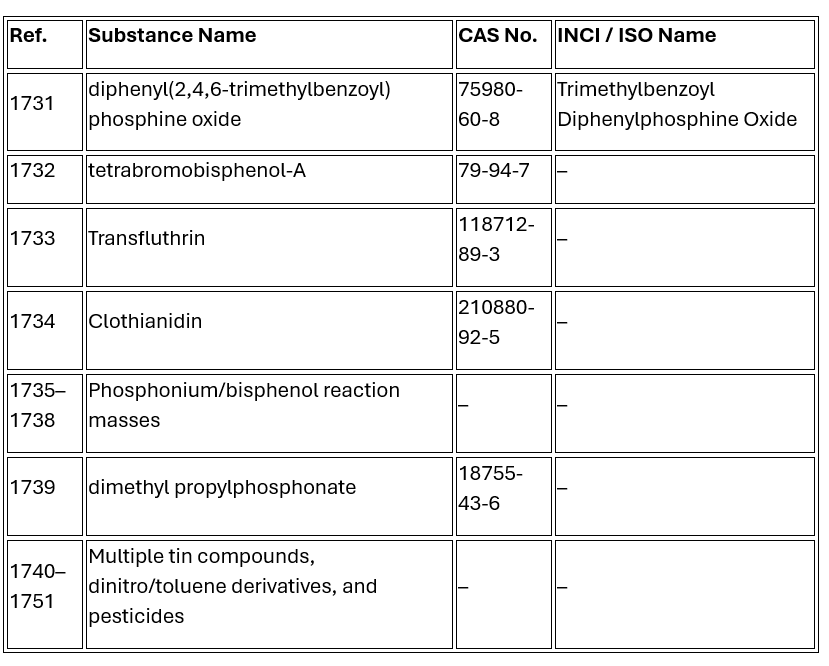
|
These substances are now fully banned, regardless of concentration or application type.
❌ Substance Removed from Annex III and Moved to Annex II
• Trimethylbenzoyl Diphenylphosphine Oxide (CAS 75980-60-8) was previously permitted in Annex III for professional use in artificial nails (Entry 311, max 5%).
• It is now fully prohibited and included in Annex II, with no exceptions for professional use.
✅ Clarification of Existing Prohibited Entries
• Cymoxanil (CAS 57966-95-7), previously listed under Annex II Entry 1580, is now updated to also reference its alternative name and second CAS number: 166900-80-7, enhancing legal clarity and enforcement accuracy.
🏛️ Legal and Regulatory Impact
• The regulation is binding across all EU Member States starting 1 September 2025.
• No derogations or exemptions have been submitted for any professional or cosmetic use.
• Products on the EU market must be reformulated or withdrawn if they contain any of the newly prohibited substances.
🧴 Industry Compliance Checklist
To ensure continued market access, cosmetic companies should take the following steps:
1. Review All Product Formulations: Identify and eliminate newly banned CMR substances.
2. Update Technical Documentation & Labels:Reflect the absence of restricted substances as required under the Cosmetics Regulation.
3. Communicate with Suppliers: Request updated safety data sheets (SDS) and reformulated raw materials, where needed.
4. Cross-Check Annex III Changes: Verify that substances previously used under concentration limits are no longer present.
⚖️ Why This Matters
The regulation supports the EU's long-term chemicals strategy for sustainability, aiming to eliminate harmful chemicals from consumer products and improve protection for human health. It also simplifies enforcement and improves market consistency across Member States.
Reach out to our regulation experts on chemical and product regulatory compliances

