Starting 1 September 2025, Switzerland will implement important revisions to its Chemicals Ordinance (ChemO) to better align with the EU’s Classification, Labelling and Packaging (CLP) Regulation and update its Candidate List of Substances of Very High Concern (SVHCs).
Key Updates in the Revision
• Annex 2: Updated classification, labelling, and packaging provisions for CLP alignment.
• Annex 3: Expanded and revised Candidate List of SVHCs, increasing total entries to 247 from 240.
New Substances Added to the SVHC List
Switzerland added seven new substances or substance groups, including:
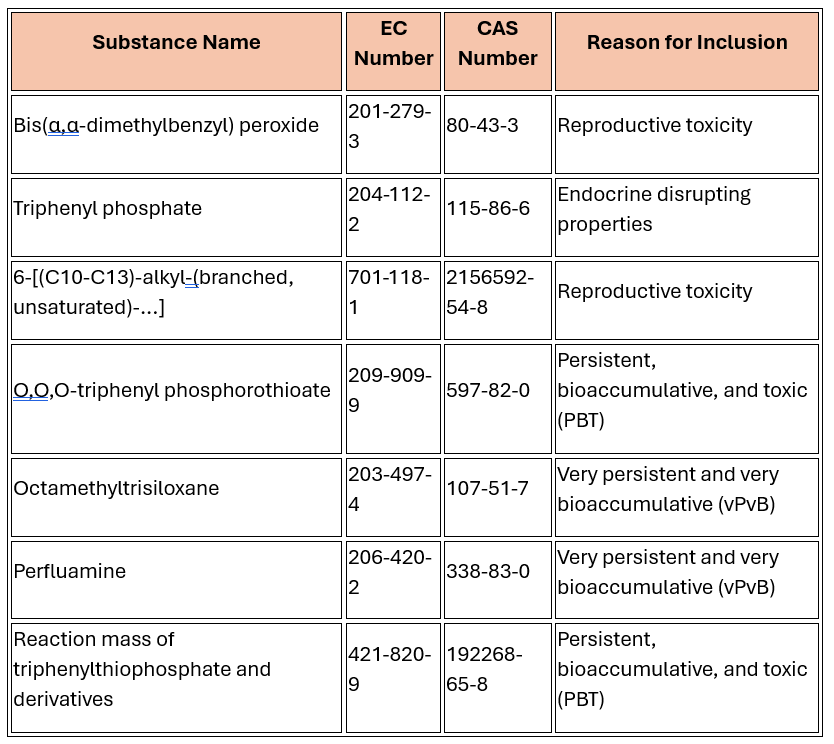
|
Supplier and Consumer Obligations
• Suppliers must provide professional users or distributors with necessary information about articles containing SVHCs >0.1%, including the SVHC name at minimum.
• Consumers can request this information free of charge, which must be provided within 45 days.
• Manufacturers must supply Safety Data Sheets (SDS) for substances and mixtures per ChemO requirements.
Transitional Arrangements for Compliance
• Substances not yet aligned with ATP-22 (Delegated Regulation 2024/2564) may remain on the market until 30 April 2026.
• Substances non-compliant with ATP-23 (Delegated Regulation 2025/1222) may be sold until 31 January 2027.
Impact on Industry
• Companies need to review products for SVHC presence.
• Update labels and SDS to meet new regulatory demands.
• Strengthen supply chain communications and meet information sharing duties.
• Track deadlines closely to avoid market interruptions.
Reference: Swiss Chemicals Ordinance Revision
Reach out to our regulation experts on chemical and product regulatory compliances

