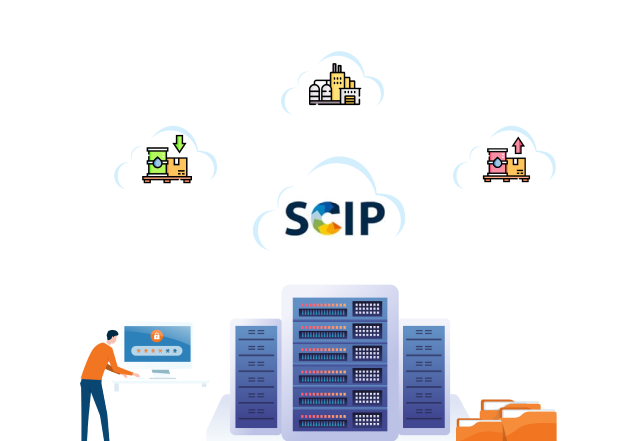
SCIP Database
As environmental concerns and sustainability become increasingly important, regulatory bodies worldwide are introducing measures to reduce hazardous substances' impact on ecosystems and human health. One of these measures is the SCIP (Substances of Concern in Products) database, which was created by the European Chemicals Agency (ECHA) as part of the Waste Framework Directive (WFD) (2008/98/EC). Adhering to SCIP is not only legally required for businesses operating within the European Union (EU), but it is also important for companies seeking to be responsible members of global supply chains and to adopt sound product stewardship practices.
The SCIP database contains information on the use of substances of very high concern (SVHCs) in articles and products above 0.1 percent weight by weight (w/w) threshold, per amendment (EU) 2018/85 in the EU market. ECHA refers to this database as Substances of Concern In articles or Products (SCIP).
The SVHCs for SCIP are based on the REACh Candidate List; SCIP is built on top of REACh. The SCIP database ensures that the substances on the Candidate list are available throughout the product life cycle, even at the waste stage. This information is publicly available to consumers and waste operators. The regulation has been in effect since 5 January 2021.

Objectives of SCIP Compliance
Transparency
SCIP compliance is designed to enhance transparency regarding the presence of Substances of Very High Concern (SVHCs) in articles throughout their lifecycle. By providing comprehensive data on SVHCs in products, SCIP plays a pivotal role in promoting transparency in supply chains. This, in turn, facilitates informed decision-making by stakeholders, including consumers, regulatory authorities, and waste treatment operators.

Safety
SCIP, by identifying articles containing SVHCs, enables businesses to implement better risk management strategies for the handling, use, and safe disposal of these substances. This, in turn, reduces potential harm to both humans and ecosystems, underscoring the tangible safety benefits of SCIP compliance.

Waste Treatment
SCIP data helps waste treatment operators identify and handle hazardous materials effectively. It facilitates proper sorting, recycling, and disposal practices, minimizing environmental contamination and health risks associated with hazardous substances.

Regulatory Compliance
Regulatory authorities can ensure safe market conditions by mandating that articles submit information on SVHCs to the SCIP database. SCIP supports enforcement efforts aimed at ensuring product safety and environmental protection within the EU market.

Sustainable consumption
SCIP supports sustainable consumption practices by providing consumers with information about the presence of Substances of Very High Concern (SVHCs) in products. This helps consumers make informed choices and encourages them to favour products that have a lower environmental and human health impact. By doing so, the regulation drives market demand for safer and more sustainable alternatives.

Promote circular economy
Contribute to developing a circular economy by promoting sustainable design, traceability, and recyclability of articles or products.
Regulatory Impact on Business
Information about the articles or products must be notified to the ECHA database in a standardized SCIP IUCLID format called SCIP dossier. Organizations placing articles or products in the EU market are required to notify the SCIP database. This affects a wide range of companies in the EU across sectors like
Manufacturers
Assemblers

Importers

Distributors and those in the supply chain
Non-compliance could result in Legal repercussions, restricted market access in the European Economic Area (EEA), disruption in the supply chain, increased audits, negative brand reputation, and even financial penalties.
SCIP implementation challenges
Here are some of the challenges that an organization may face when implementing the SCIP database.
For detailed insight, read this blog.
-
 Substance Identification: Identifying substances of very high concern used in the articles or products requires a full material declaration, which can be complex, especially when there are fewer resources involved.
Substance Identification: Identifying substances of very high concern used in the articles or products requires a full material declaration, which can be complex, especially when there are fewer resources involved.
-
 Product hierarchy: Substances of concern may be at higher nodal levels in complex articles or products, making it difficult to identify the product hierarchy.
Product hierarchy: Substances of concern may be at higher nodal levels in complex articles or products, making it difficult to identify the product hierarchy.
-
 Supply chain complexities: Communication with suppliers to ensure that substances in the supply chain are compliant can be difficult, especially if the supply chain is complex, non-transparent, or spans multiple countries. Communication of information throughout the supply chain can be challenging. Moreover, it is also necessary that supplier information received is audited and consolidated to guarantee accuracy, which can be tedious and time-consuming.
Supply chain complexities: Communication with suppliers to ensure that substances in the supply chain are compliant can be difficult, especially if the supply chain is complex, non-transparent, or spans multiple countries. Communication of information throughout the supply chain can be challenging. Moreover, it is also necessary that supplier information received is audited and consolidated to guarantee accuracy, which can be tedious and time-consuming.
-
 Collaboration between stakeholders: To execute the regulatory process, stakeholders in larger organizations need to collaborate and communicate effectively. Delays in communication and inconsistent follow-ups can disrupt the activity and affect the outcome.
Collaboration between stakeholders: To execute the regulatory process, stakeholders in larger organizations need to collaborate and communicate effectively. Delays in communication and inconsistent follow-ups can disrupt the activity and affect the outcome.
-
 Manual Submissions: If distributors, assemblers, and vendors decide on manual submissions, it can introduce multiple layers of complexity, including data collection from the supply chain, standardization, data confidentiality, monitoring, and finally, the generation of SCIP Dossiers in IUCLID format can become a staggering task.
Manual Submissions: If distributors, assemblers, and vendors decide on manual submissions, it can introduce multiple layers of complexity, including data collection from the supply chain, standardization, data confidentiality, monitoring, and finally, the generation of SCIP Dossiers in IUCLID format can become a staggering task.
APAs 6 steps to help you comply with SCIP Notifications
For detailed information on how to ‘Simplify SCIP Notifications,’ read here. You can leverage our services and software tools, which can assist you in preparing the SCIP Dossier with ease, submitting it promptly prior to the deadlines.

Risk-based Analysis & Identification of products meeting SCIP requirements

Collaboration and Communication with the Suppliers throughout the supply chain.

SCIP Dossier Preparation in IUCLID format (both Online & Manual) through Simplified SCIP Notification and SCIP Referencing for Complex Dossiers.

Registration and Submission into the ECHA SCIP database

Software tools like ‘Greencheck’ for enabling automated submission (system to system

Monitoring for the latest updates.