Regulatory Context
Japan continues to strengthen its chemical safety framework by imposing bans on specific per- and polyfluoroalkyl substances (PFAS), reflecting global efforts to mitigate environmental and health risks associated with these persistent chemicals.
Key Phase-Out Deadlines
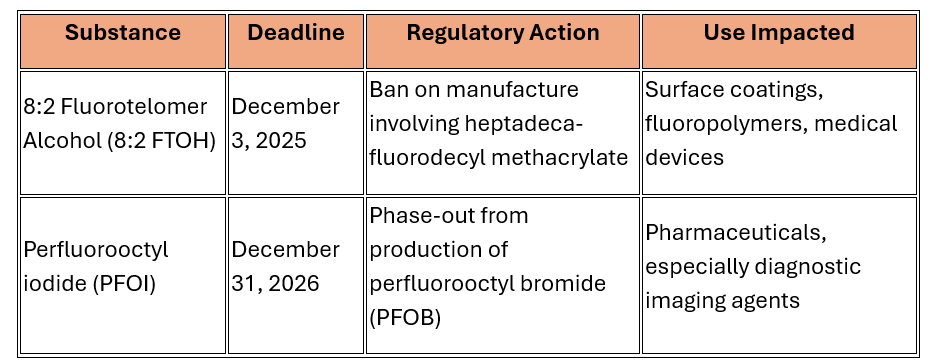
|
Scientific & Regulatory Rationale
• Environmental Persistence: Both 8:2 FTOH and PFOI are highly persistent, bioaccumulative PFAS chemicals that pose long-term ecological risks.
• Health Concerns: Linked to endocrine disruption, reproductive and developmental toxicity, and potential carcinogenicity.
• International Alignment: Consistent with Stockholm Convention goals and other global PFAS regulatory initiatives.
Industry Sectors Affected
• Medical Devices: Use of PFAS-based hydrophobic and performance coatings.
• Pharmaceuticals: Utilization of PFOB as a contrast agent in diagnostic imaging.
• Electronics & Specialty Chemicals: PFAS derivatives employed for chemical stability and resistance.
Recommended Business Actions
• Inventory & Usage Assessment: Identify all materials and processes involving 8:2 FTOH and PFOI.
• Alternative Evaluation: Research safer chemical substitutes with equivalent functionality.
• Regulatory Monitoring: Track updates and guidance from Japan’s Ministry of Economy, Trade and Industry (METI).
• Supply Chain Engagement: Collaborate with vendors to secure compliant raw materials.
• Transition Planning: Prepare phase-out schedules and, if needed, submit authorization requests timely.
Reference: METI Press Release
Reach out to our regulation experts on chemical and product regulatory compliances

