European Chemicals Agency (ECHA) recommended that the European Commission add four chemicals to the REACH Authorisation List (Annex XIV). If accepted, their use in the EU/EEA would face stricter regulatory control, requiring companies to obtain authorisation to continue using them.
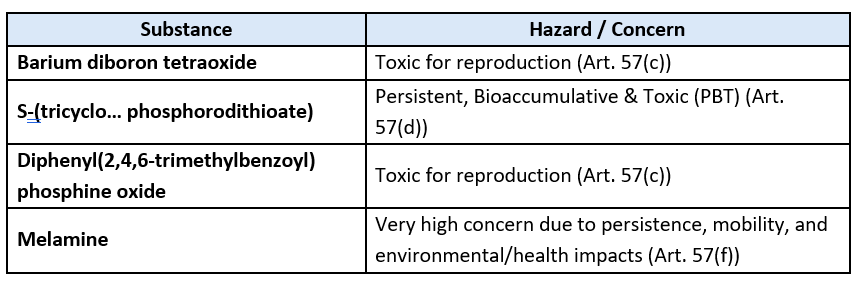
The Four Substances & concerns
|
ECHA identified these substances as "highest priority" under its Substances of Very High Concern (SVHC) prioritisation process. This marks the first new authorisation recommendation in more than 2.5 years, signaling renewed regulatory momentum.
Definition of Authorisation Recommendation
These substances are not banned yet, but they are proposed for tighter control. The European Commission will decide:
• Whether to officially list them in Annex XIV
• A sunset date, after which unapproved use is banned
• Any exempted uses
• Whether authorisation is required for specific applications
If listed, companies must demonstrate either:
1. Risks are adequately controlled, or
2. Socio-economic benefits outweigh risks and no safer alternative exists
Industries such as automotive, electronics, plastics, coatings, adhesives, lubricants, and consumer goods may need substitution, reformulation, or discontinuation of affected applications.
Industries & Applications Likely to Be Affected
• Coatings, paints, adhesives, sealants, polymers
• Inks, toners, printing & UV-curable products (photoinitiators)
• Lubricants, greases, metalworking fluids
• Plastics and molded components (auto, electronics, toys, consumer goods)
• Furniture & construction materials (melamine resins, laminates, adhesives)
Given the broad use of these substances, both raw-material suppliers and finished-goods manufacturers should assess their exposure.
Recommended Action Plan for Companies
1. Review Inventory & Supply Chain
• Check materials, coatings, additives, inks, lubricants, resins, and sealants
• Identify any presence of the four substances, even at low levels
2. Check Possible Exemptions
• Some intermediate uses (e.g., melamine in closed processes) may be exempt
• Evaluate if your application requires full authorisation
3. Plan Substitution
• Prioritise safer alternatives for high-volume or high-risk uses
• Start reformulation early to avoid disruptions
4. Prepare for Potential Authorisation
• Gather exposure data, use scenarios, and alternatives assessments
• Prepare socio-economic justification if needed
5. Communicate Across the Supply Chain
• Inform suppliers and customers
• Update SDS, procurement documentation, and declarations
6. Monitor EU Regulatory Decisions
• Track Commission decision
• Follow final inclusion, sunset dates, exemptions, and application deadlines
Importance of the substance recommendation
This move strengthens the EU’s focus on SVHCs, especially substances that are toxic, persistent, bioaccumulative, mobile or with high long-term risks. Early preparation can help global manufacturers avoid market disruptions, customs issues, and last-minute reformulation costs.
Reference: Newly Added SVHC's
Reach out to our regulation experts on chemical and product regulatory compliances

