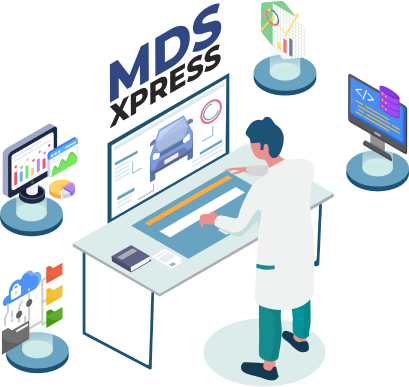
IMDS Reporting
Submitting Material Data Sheets (MDS) to the International Material Data System (IMDS) can be difficult for automotive manufacturing companies. Several challenges are involved, such as dealing with a low supplier response rate, creating complex tree structures for large assemblies, and identifying missing child MDS in the assembly. However, MDS Xpress can revolutionize the way you prepare for IMDS reporting
Why Choose MDS Xpress?
Effortless IMDS Reporting
With MDS Xpress, Submitting Material Data Sheets to the IMDS platform is now easier and more efficient. Our user-friendly interface and powerful features simplify the entire process.

Comprehensive
Compliance
Using MDS Xpress, ensure 100% compliance with global regulations like ELV, RoHS, RRR, and GADSL. Identify unreported Material Data Sheets within your Bill of Materials (BOM).

Accuracy Assurance
MDS Xpress provides robust validation mechanisms to ensure the accuracy of your MDS data, including crucial information for RRR (Recycle, Reuse, Recover) calculation, minimizing the risk of non-compliance.

Effortless IMDS Reporting
Automate tedious tasks such as building tree structures for large assemblies and communicating with suppliers to save valuable time and resources.
Capabilities of MDS - Xpress

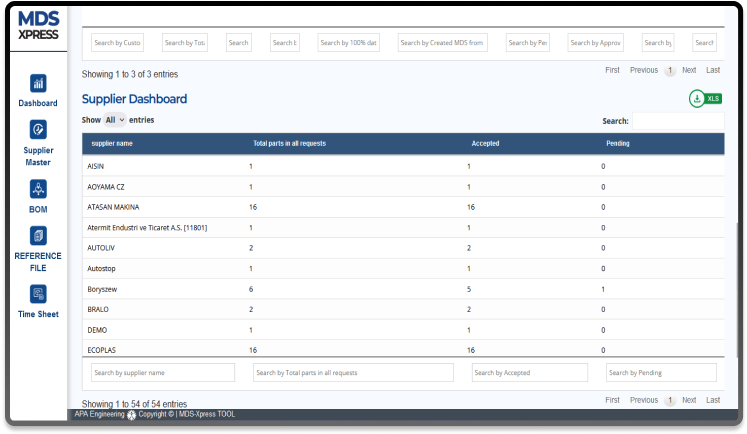
Dashboards
Gain valuable insights with dashboards for Suppliers, Customers, and Material data.

BOM Analysis
Effortlessly identify unreported Material Data Sheets (MDS) in a Bill of Materials (BOM) for comprehensive compliance.

MDS Analysis & Validation
Conduct a comprehensive analysis of multiple substances and CAS numbers. Validate MDS data with respect to several IMDS recommendations that will help users to take a decision on accepting or rejecting the MDS
Building Tree Structures
Efficiently generate error-free IMDS tree structures for large assemblies from imported BOMs, eliminating manual effort.

RRR Calculation
To minimize overall waste generation and environmental impact, calculate vehicle-level recyclability, recoverability, and reusability based on ISO22628 standards.

Automate SCIP dossier submission
Save your time and simplify the submission of SCIP dossier to ECHA by automating the process.

CAMDS Support
The software can extract data from CAMDS PDF files to help with regulatory compliance for China Automotive Material Data System.

Supplier Communication
Automate email communication and reminders to facilitate faster submission processes and reduce follow-ups with suppliers regarding unreported and rejected MDS.
Maximize your business benefits
-
 Cost Savings: Cut costs by 30% yearly through automated IMDS submissions.
Cost Savings: Cut costs by 30% yearly through automated IMDS submissions. -
 Time Savings: Automated tree structure analysis is a time-saving process that can reduce analysis time by up to 70%.
Time Savings: Automated tree structure analysis is a time-saving process that can reduce analysis time by up to 70%. -
 Accuracy and Efficiency: Reduce process costs by 30% by streamlining submissions and eliminating manual tasks.
Accuracy and Efficiency: Reduce process costs by 30% by streamlining submissions and eliminating manual tasks. -
 ERP Integration: Integrate with your in-house or third-party ERP systems to minimize data redundancy.
ERP Integration: Integrate with your in-house or third-party ERP systems to minimize data redundancy. -
 Faster Submission: Expedite supplier submissions with automated communication, ensuring a more responsive and efficient process.
Faster Submission: Expedite supplier submissions with automated communication, ensuring a more responsive and efficient process.

Reach Out for Error-Free IMDS
Submissions Now
Are you tired of manual processes and inefficiencies holding you back? It's time to experience the power of automation and unlock significant cost savings while streamlining your IMDS submission process. Contact us now to learn more about how our solution can revolutionize your approach to IMDS compliance.