South Korea’s Ministry of Food and Drug Safety (MFDS) has issued a draft amendment to the Enforcement Rule of the Cosmetics Act, significantly altering how cosmetics are labeled and classified. The proposed revisions eliminate the legal distinction between “natural” and “organic” cosmetics, folding these categories into the broader umbrella of functional cosmetics.
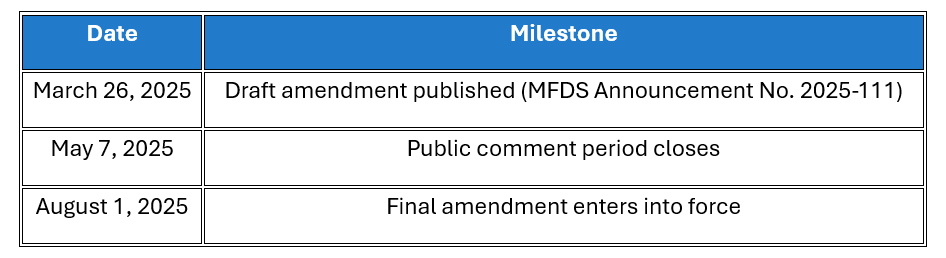
📆 The changes are set to take effect on August 1, 2025, with a public comment period open until May 7, 2025.
Key Regulatory Changes in the Draft Amendment
Under MFDS Announcement No. 2025-111, the proposed amendment includes the following major changes:
❌ Abolition of “natural” and “organic” classifications
These categories will be removed as legally distinct product types and absorbed into the functional cosmetics category.
🏷️ Labeling and advertising revisions
All references to “natural” and “organic” will be prohibited on cosmetic labels, packaging, and promotional materials.
✅ Unified regulatory standards
Functional cosmetics will now serve as the single regulated category for all previously designated products.
Implementation Timeline
|
Impact on Cosmetic Companies
Cosmetic manufacturers, importers, and marketers in Korea should prepare for regulatory changes that affect:
• Product Classification
Products previously labeled as “natural” or “organic” must be reclassified under the functional cosmetics category.
• Labeling Compliance
All packaging and marketing content must be updated to remove disallowed terminology by the effective date.
• Advertising Strategy
Claims tied to natural or organic features must be revised or substantiated under new functional criteria.
Recommended Actions for Businesses
To ensure uninterrupted product distribution and avoid regulatory penalties, companies are advised to:
🧾 Audit current product lines to identify items labeled as natural or organic
🖋️ Revise labels, claims, and advertisements to comply with the amended rules
📣 Engage in the public comment process to raise industry concerns or request clarification
🗂️ Update internal regulatory and compliance documentation ahead of the August 2025 deadline
Early preparation will help reduce supply chain disruptions and ensure continued market access.
Reference: MFDS Announcement No. 2025-111 – Draft Amendment to Cosmetics Act Enforcement Rule
Reach out to our regulation experts on chemical and product regulatory compliances

